What is REBOOT?
REhabilitation after BOne marrOw Transplant to improve patient outcomes
Who is participating?
Patients who are 18 years or over
Able to ambulate independently
30 days after an autologous or allogeneic bone marrow transplant
Able to provide consent and are proficient in English to understand exercise testing and training instructions
The main reasons why somebody may not fit the study criteria are:
Re-occurring malignancy
Severe or unstable neurological, cardiorespiratory or musculoskeletal disease or mental illness that might compromise ability to perform exercise
Unstable psychiatric or cognitive disorders
ECOG performance status >2
Can I sign up to the trial?
Eligible participants will be referred by the clinical team need at one of the three participating sites. Interested people should discuss with a member of their clinical team.
What is involved?
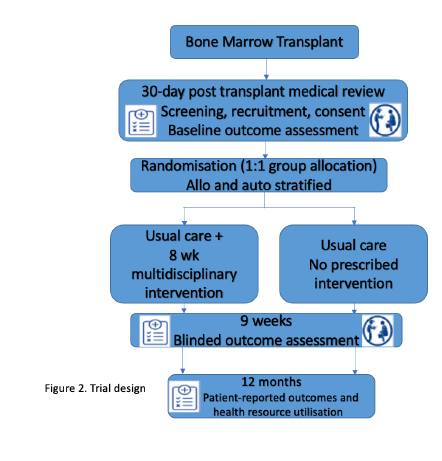
1. Signing up to the study: At the 30-day post-transplant medical review, a member of the team will discuss the study and provide a person with written information if the person is interested in taking part. If eligible and willing to take part, and if clinically indicated, a person will be recruited into the study.
The person will be asked to provide written informed consent, and will undertake a baseline assessment.
2. Randomisation: After the baseline assessment, the person will be allocated randomly (by chance using a computer programme) to one of two sleep study types:
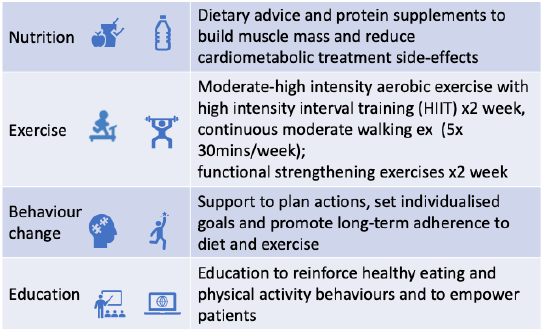
1. Usual care and a multidisciplinary intervention (exercise, nutrition, behaviour change)
2. Usual care and no additional prescribed intervention
3. Treatment: The treatment period (either intervention or usual care) will continue for 8 weeks.
4. Follow Up: From week 9 onwards, the person will be asked to return for a follow-up assessment.
All participants will be asked to complete assessments again 12 months.
Trial Design
Components of the Intervention
More details
Participant data will be de-identified when collected with the use of a unique participant identification number (trial identifier).
All participants will have access to their usual Clinical team throughout the trial period as they would under normal circumstances.
The trial is registered at ClinicalTrials.gov. Ethical approval for all sites has been granted.